Abstract
Introduction: Venous thromboembolism (VTE) is common in cancer patients and associated with adverse consequences. Risk factors for cancer-associated VTE are multifactorial and a validated tool, the Khorana risk score (KRS), is recommended by guidelines to determine risk. This study evaluates contemporary rates of VTE in real world settings using this risk score.
Methods: The Optum©'s Clinformatics® Data Mart database (01/01/2012 - 09/30/2017) was used to select adult patients with ≥1 hospitalizations or 2 outpatient medical claims with a cancer diagnosis (index date) who were initiated on cancer therapy (e.g., chemotherapy or radiation therapy) within 45 days of the index date. To be included in this study, patients were also required to meet the following criteria: (1) continuous eligibility for ≥6 months before the index date (baseline period), (2) no VTE event during the baseline period, (3) no use of anticoagulants prior to the index date or up until a VTE event during the follow-up, (4) no evidence of a major surgery after the index date, (5) ≥1 laboratory test result for hemoglobin, leukocyte, and platelet counts within 28 days before cancer treatment initiation. The KRS was calculated using the index cancer site, body mass index, and laboratory test results prior to treatment initiation (i.e., hemoglobin, leukocyte, and platelet counts). Based on the risk score, patients were classified into cohorts of 0, 1, 2 or ≥3 KRS. The main outcome was a VTE; defined as a primary VTE diagnosis during a hospitalization or a secondary VTE diagnosis during a hospitalization followed by either 1) another hospitalization or outpatient visits with a diagnosis (primary or secondary) of VTE or 2) an anticoagulant dispensing within 30 days after the discharge date of the first hospitalization. Kaplan-Meier (KM) analyses were used to evaluate time to first VTE event. Patients without an event were censored at the end of the observation period (i.e., the earliest date between the end of data availability, death, end of insurance coverage, or 12 months post-index). KM rates were reported at 12 months and cohorts were compared using adjusted Cox proportional hazards models controlling for the following baseline characteristics: age, sex, insurance type, year and month of index date, Charlson comorbidity index (CCI) score, Elixhauser comorbidities with a proportion ≥5%, index cancer type, and healthcare resource utilization and costs.
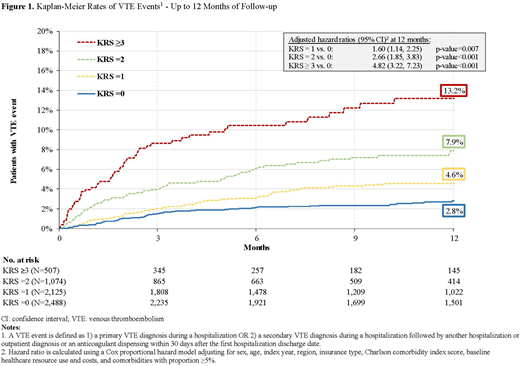
Results: The study population comprised 6,194 patients (KRS=0: 2,488; KRS=1: 2,125; KRS=2: 1,074; KRS≥3: 507). Patients were, on average, 68 years old, 48% female, and mean CCI score ranged from 1.1 to 1.4 for 1 to ≥3 KRS. The mean observation period was 9.6, 8.7, 8.0, and 6.9 months for KRS cohorts of 0, 1, 2, and ≥ 3, respectively. The 12-month cumulative VTE rates were 2.8% (KRS=0), 4.6% (KRS=1), 7.9% (KRS=2), and 13.2% (KRS≥3); with an associated mean (median) time to VTE event of 3.0 (2.0), 3.6 (3.0), 3.6 (2.7), and 2.3 (1.6) months for KRS cohorts of 0, 1, 2, and ≥ 3, respectively (Figure 1). The adjusted hazard ratio (95% confidence interval) at 12 months using the KRS=0 cohort as a reference cohort was 1.60 (1.14, 2.25) for KRS=1 vs 0; 2.66 (1.85, 3.83) for KRS=2 vs 0; and 4.82 (3.22, 7.23) for KRS ≥3 vs 0 (all p-values<0.05).
Conclusions: This real-world retrospective analysis found that the rate of VTE events among newly diagnosed cancer patients who were initiated on cancer therapy significantly increased with VTE risk level based on the KRS. Over 25% of patients in the observed cohort have KRS≥2 and are 3 times more likely to develop VTE from the time of cancer diagnosis. These findings further validate the KRS in contemporary oncology patients and support ongoing studies of thromboprophylaxis in patients with a score of 2 or higher.
Khorana:Sanofi: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Janssen: Consultancy. Kuderer:Celldex: Consultancy; Mylan: Consultancy, Other: Travel, Accommodations, Expenses; Coherus Biosciences: Consultancy, Other: Travel, Accommodations, Expenses; Halozyme: Consultancy; Myriad Genetics: Consultancy; Pfizer: Consultancy; Janssen Scientific Affairs, LLC: Consultancy, Other: Travel, Accommodations, Expenses. Milentijevic:Janssen Scientific Affairs, LLC: Employment, Equity Ownership. Germain:Janssen Scientific Affairs, LLC: Research Funding. Laliberté:Janssen Scientific Affairs, LLC: Research Funding. Le:Janssen Scientific Affairs, LLC: Research Funding. Lefebvre:Janssen Scientific Affairs, LLC: Research Funding. Lyman:Generex Biotechnology: Membership on an entity's Board of Directors or advisory committees; Halozyme; G1 Therapeutics; Coherus Biosciences: Consultancy; Amgen: Other: Research support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal